Maria McNamara on Seeing the Ancient World in Color
Listen to the podcast here or wherever you listen to podcasts.
Maria McNamara is Professor of Paleontology at University College Cork in Ireland. Her research focuses on the preservation of non-biomineralized ‘soft’ tissues, such as skin, muscle, and internal organs. She examines such tissues in exceptionally well-preserved fossils, including many that retain color-producing structures at the micron scale.
In the image, she is holding samples of pterosaur feathers on a microscope mount.
Courtesy of Daragh McSweeney/Provision
Podcast Illustrations
All images courtesy of Maria McNamara unless otherwise indicated.
Exceptionally well-preserved fossils
In order to see the fossilized structures at the micron scale, the fossils must be exceptionally well preserved. As Maria McNamara discusses in the podcast, soft tissues can be preserved in extraordinary detail when certain conditions prevail. These include oxygen-starved settings and rapid burial sites. As of 2022, about 600 sites of exceptional fossil preservation are known around the world. Such deposits are called Lagerstätten. Well-known examples include the Cambrian Burgess Shale, the Carboniferous Mazon Creek, and the Eocene Green River Formation.
The image shows examples of exceptionally well-preserved fossils. (a) Phosphatized embryo Megasphaera from the Ediacaran Doushantuo Formation, China. (b) Silicified multicellular alga Wengania from the Ediacaran Doushantuo Formation, China. (c) Pyritized tubular metazoan Conotubus from the Ediacaran Dengying Formation, China. (d) Ediacara-type fossil Swartpuntia from the Ediacaran Nama Group, Namibia. (e) Aluminosilicified carbonaceous compression of arthropod Marrella from the Cambrian Burgess Shale, Canada. (f) Oxidized compression of a ctenophore (“comb jelly”) from the Cambrian Qiongzhusi Formation (Chengjiang Biota), China (Yunnan University specimen RCCBYU 10217). (g) Carbonaceous compression of eurypterid from the Silurian Bertie Waterlime (Fiddlers Green Formation), US. (h) Silicified mayfly from the Miocene Barstow Formation, US. (i) Carbonaceous compression of insect Fulgora from the Eocene Green River Formation, US. (j) Fish from the Eocene Green River Formation, US.
Muscente et al. (2017), Gondwana Research 48, 164
Colors from Pigments
Pterosaur melanosomes
Scanning electron micrographs of the soft tissues of a pterosaur’s fossilized skull reveal that different types of feathers contained different shapes of pigment-bearing melanosomes. As Maria McNamara explains in the podcast, melanosome shapes are linked to color. In the top row, whisker-like simple filaments contain elongated melanosomes, suggesting darker colors. In the bottom row, more complex branched feathers contain ovoid melanosomes, pointing to brighter yellows or reds. Scale bars all represent 2 microns.
Cincotta, A. et al. (2022), Nature 604, 684
Structural Colors
Structurally colored fossil moths from the Eocene
Structurally colored scales in fossil moths with striking metallic hues from 47-million-year-old oil shales in Germany. The original colors were altered during fossilization. Preserved details in the scales allow reconstruction of the original colors and show that the dorsal surface of the forewings was yellow-green. The optical properties of the scales strongly indicate that the color functioned as a warning signal during feeding but was cryptic when the moths were at rest. (A–C) Light micrographs with details of areas indicated in (B, C). (D–J) Scanning electron micrographs of scales. (D) Surface showing longitudinal ridges and transverse cross-ribs and micro-ribs. (E) Two overlapping scales showing windows, perforations, and internal laminae of the upper, fractured scale. Arrow indicates densely packed bead- to rod-like spacers in the uppermost internal lamina. (F, G) Windows and perforations in proximal (F) and distal (G) parts of a scale. (H) Oblique fracture through scale showing successive internal laminae. (I) Surface of internal lamina showing perforations and bead-like spacers. (J) Horizontally fractured scale showing connective tissue (trabeculae, fractured and lying parallel to the scale surface) and reticulate basal lamina with, inset, intact vertically orientated trabeculae. Scale bars: (A), 5 mm; (B, C), 1 mm; (D, E, H, J) (including inset), 2 mm; (F, G, I), 1 mm.
McNamara, M.E. et al. (2011), PLoS Biology 9(11): e1001200
Transmission electron micrographs of the fossil moth multilayer reflector
(A) Vertical longitudinal section through a stack of four scales; scales are fractured locally. r, resin; s, sediment. (B) Detail of multilayer nanostructure in a longitudinal section through a single scale. (C) Transverse section through a scale showing broad ridges and the concave geometry of the interridge surface and of the underlying multilayer structure. Scale bars: (A, C), 1 mm; (B), 500 nm.
Reconstruction of the original colors of the dorsal
surface of the fossil lepidopterans.
The dominant hue of interior zones of the fossil wings are blue today (when viewed in air) but were originally yellow-green. The edges of the wing were originally green-cyan and blue, and the outer wing margin, brown.
McNamara, M.E. et al. (2011), PLoS Biology 9(11): e1001200
Pleistocene Beetle
In the podcast, Maria McNamara describes structural colors and photonic crystals in 700,000-year-old subfossils from the Pleistocene glacial deposits. These images show subfossil weevil scales from Swiss glacial deposits captured using a light microscope (a, b, e, f) and a scanning electron microscope (c, d, g, h). The images in the right column are enlarged and rotated versions of the regions bounded by white boxes in the corresponding image in the left column. The light micrographs show the preservation of scales with bright blue, green, and yellow hues, while electron micrographs reveal three-dimensional photonic nanostructures.
McDonald, L.T., et al. (2020), Biol. Lett. 16: 20200063
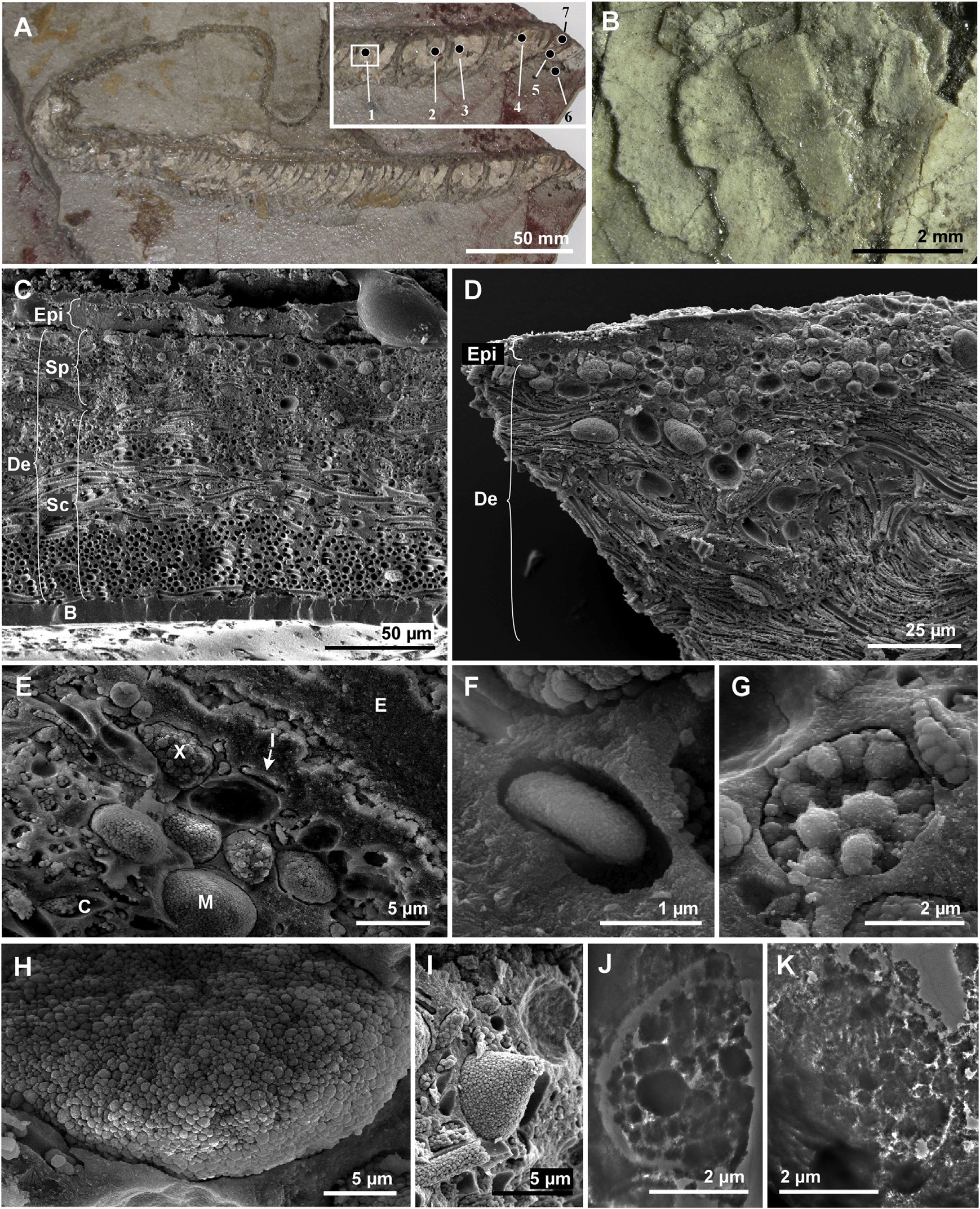
Fossil skin from a 10 million-year-old colubrid snake found in Teruel, Spain
The color of a given region of skin depends on the relative abundance of the various pigment cells. (A) The entire specimen; inset shows anterior. Cream-colored material is fossil skin. Numerals 1–7 show the sample locations. (B) Overlapping scales. (C–E) Scanning electron micrographs of fractured vertical sections through the skin, showing epidermis (Epi), dermis (De), basement membrane (B), chromatophores (pigment cells - iridophores [I], melanophores [M], and xanthophores [X]), stratum spongiosum (Sp), stratum compactum (Sc), and collagen fibers (C). (F–I) Details of iridophore (F), xanthophore (G), and melanophores (H and I). (J and K) Transmission electron micrographs of xanthophore (J) and melanophore (K).
McNamara, M.E. et al. (2016), Current Biology 26, 1075
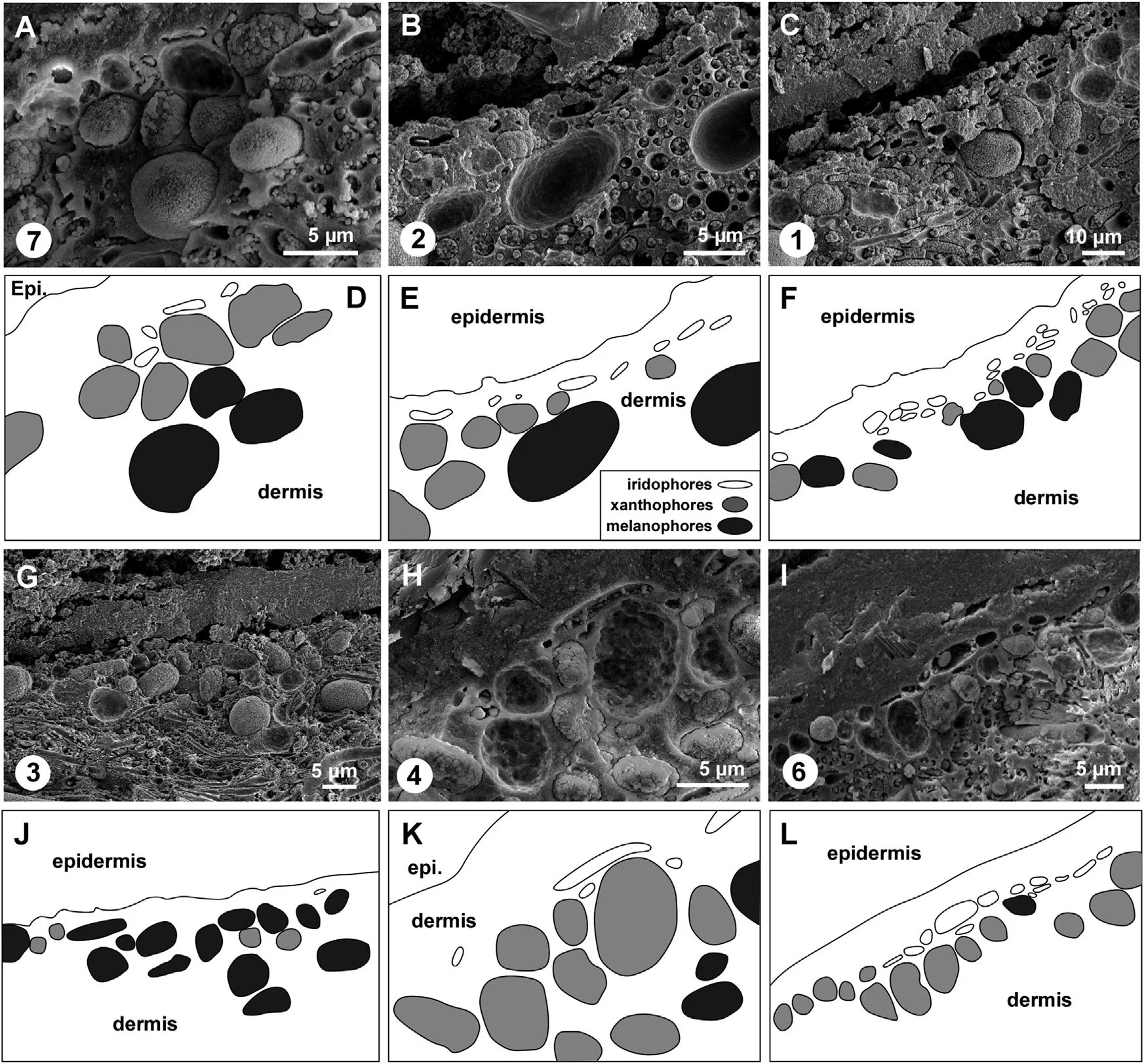
Variation in the relative abundance of different pigment cells in skin from different body regions of the Teruel snake
Encircled numerals correspond to sample numbers in A of the previous set of images. (A–C and G–I) Scanning electron micrographs of vertical sections through the fossil skin. (A) Abundant xanthophores; common iridophores and melanophores. (B) Common iridophores and xanthophores; occasional melanophores. (C) Abundant iridophores; common melanophores and xanthophores. (G) Abundant melanophores; common xanthophores; rare iridophores. (H) Abundant xanthophores; occasional iridophores; rare melanophores. (I) Abundant xanthophores and iridophores; rare melanophores. (D–F and J–L) Interpretative drawings corresponding to (A–C and G–I). Epi., epidermis.
Color reconstruction of the Teruel fossil snake
(A) Schematic representation of the relative abundance and position of pigment cells in samples of skin from different body regions. (B) Artist representation of the color reconstruction.